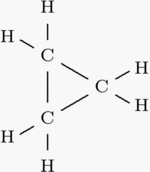
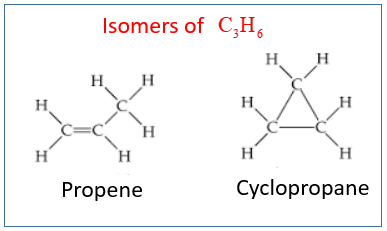
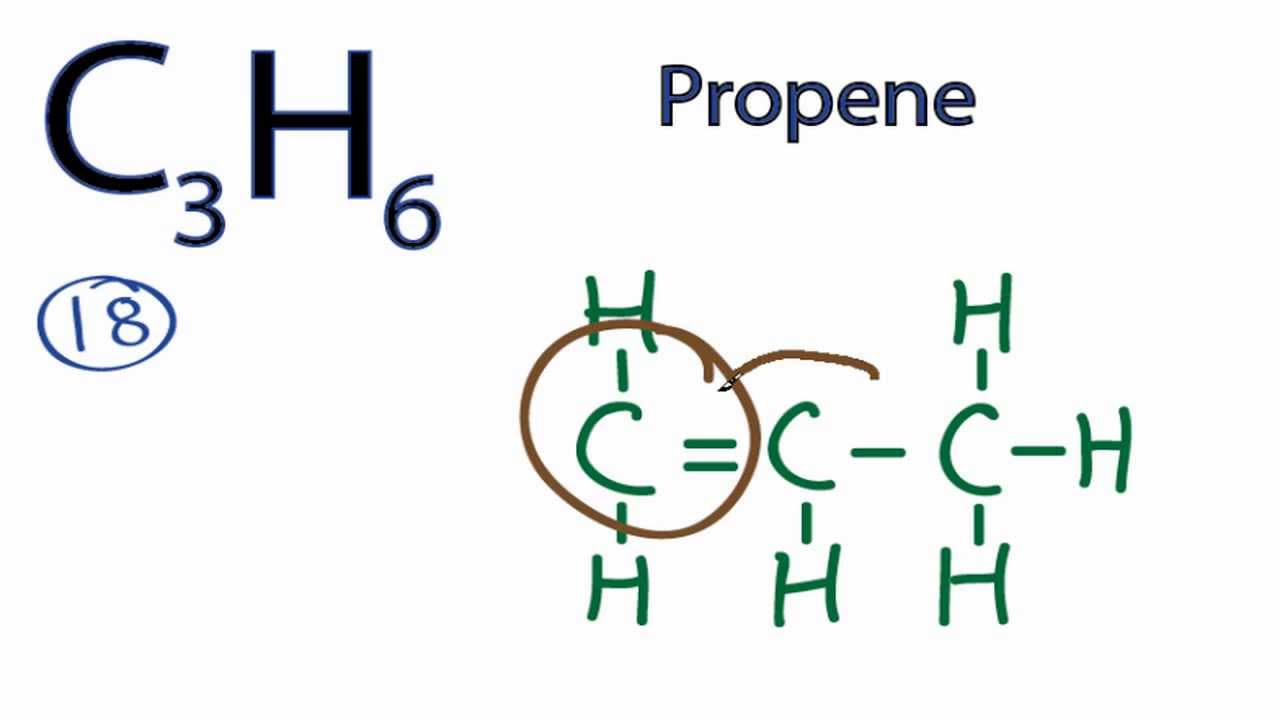
C3h6 Isomers
First check the victim for contact lenses and remove if present.
C3h6 isomers. A step by step explanation of how to draw the c3h6 lewis structure. Do not put any ointments oils or medication in the victim s eyes without specific instructions from a physician. Constitutional isomers are molecules that have the same molecular formula but they have a different connectivity of atoms in the molecules. Since the formula is deficient in two hydrogen atoms relative to a saturated hydrocarbon all of the structures need a double bond or a ring.
It has one double bond and is the second simplest member of the alkene class of hydrocarbons. Nopropane is c3h8cyclopropane is c3h6 therefore they have. Suppose we have a molecule with a molecular formula c2h6o. The formula c3h6 is associated with two isomers 1 propane and 2 cyclopropane.
The rate law is first order in cyclopropane and the rate constant is 6 0x10 4 s 1 at 500c. Building the lewis structures for this molecule reveals the two possibilities constitutional isomers read more. Are propane and cyclopropane isomers. It s best if i illustrate the idea of constitutional isomers with an example.
This set index page lists chemical structure articles associated with the same molecular formula. Flush victim s eyes with water or normal saline solution for 20 to 30 minutes while simultaneously calling a hospital or poison control center. A if the initial concentration of cyclopropane is 0 0226 m what is the concentration after 525 s. Isomers have different formulas.
If a coefficient of 1 is required choose blank for that box. Saturated hydrocarbons are generally unreactive except in the presence of. If an internal link led you here you may wish to change the link to point directly to the intended article. I will assume that you want pretty much everything including chemical and structural isomers.
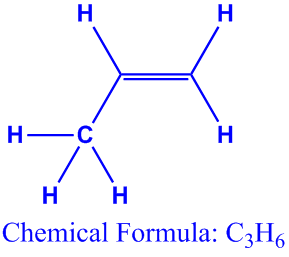
Propene also known as propylene or methyl ethylene is an unsaturated organic compound with the chemical formula displaystyle ce c3h6. I o o o i. Cyclopropane c 3 h 6 is converted to its isomer propene ch 2 chch 3 when heated. C3h6 c22h24 ch4 no2.
The hydrocarbon c2h4 is a member of the series.














.png)