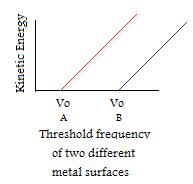
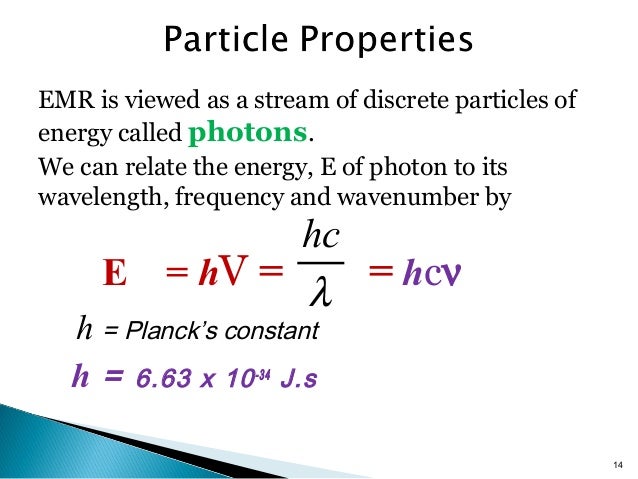
E Hv Chemistry

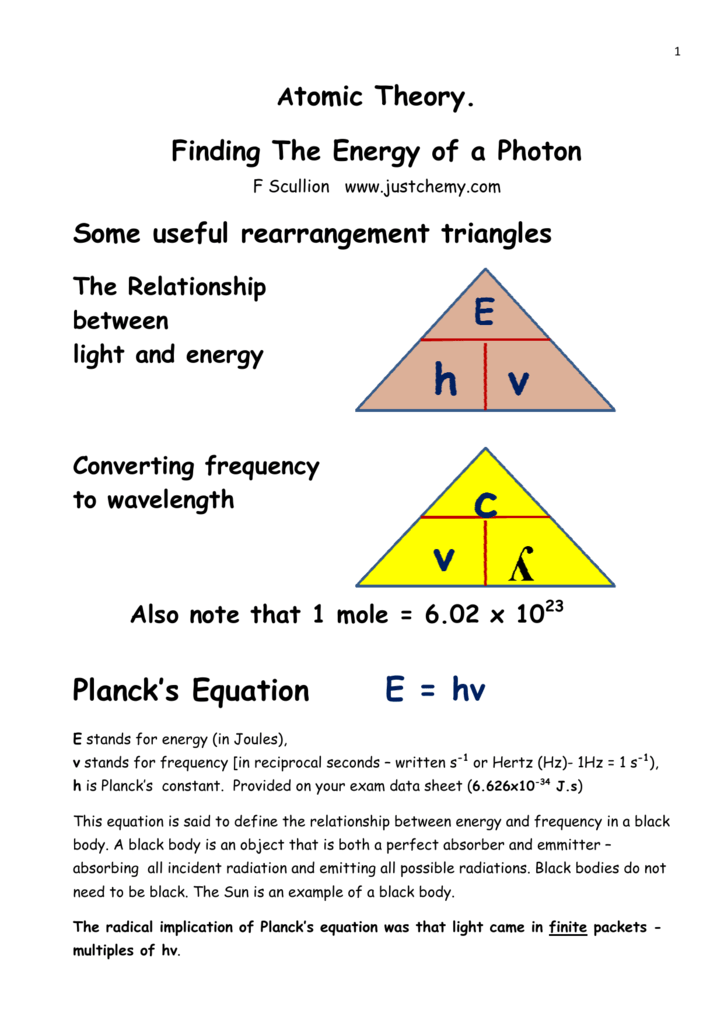
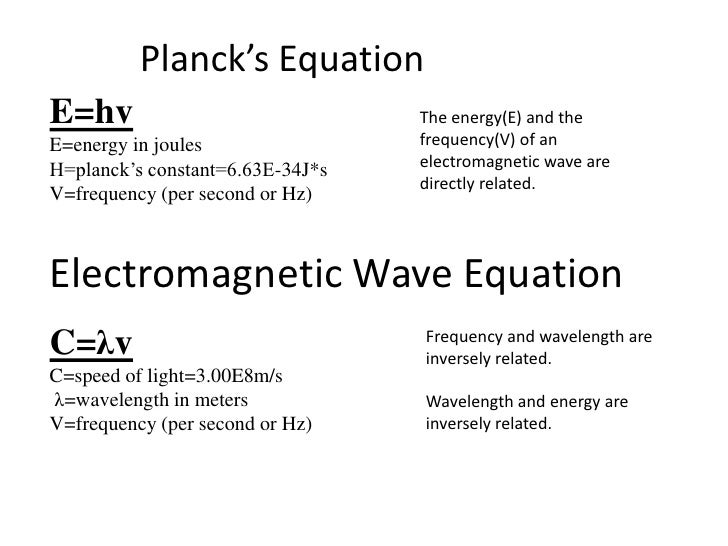
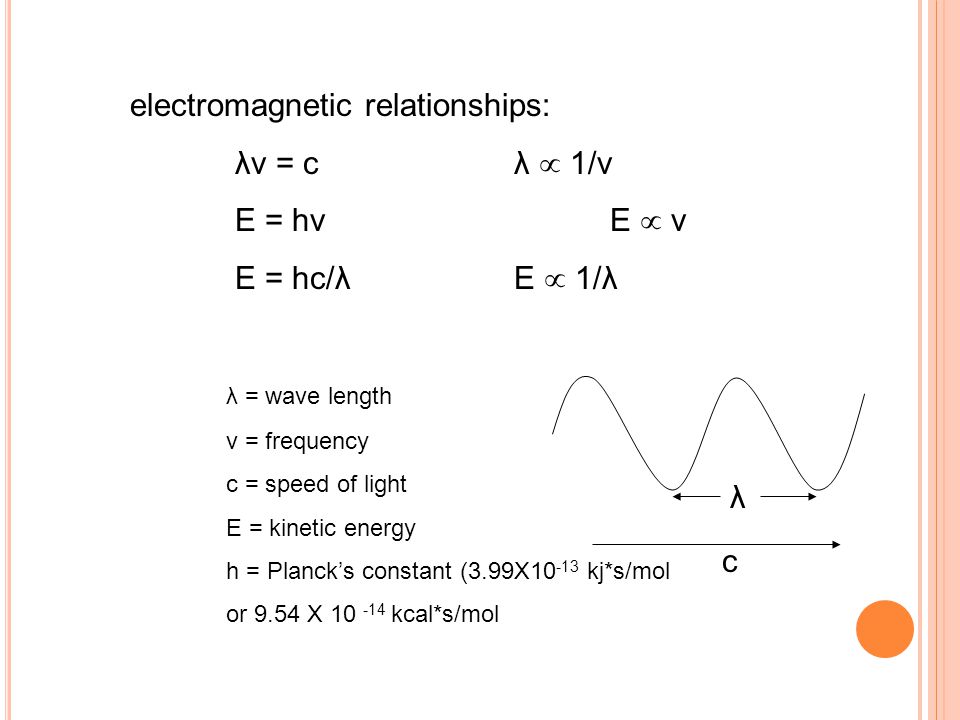
The capital e represents energy the h represents planck s constant and the v is the greek letter nu which symbolizes frequency.
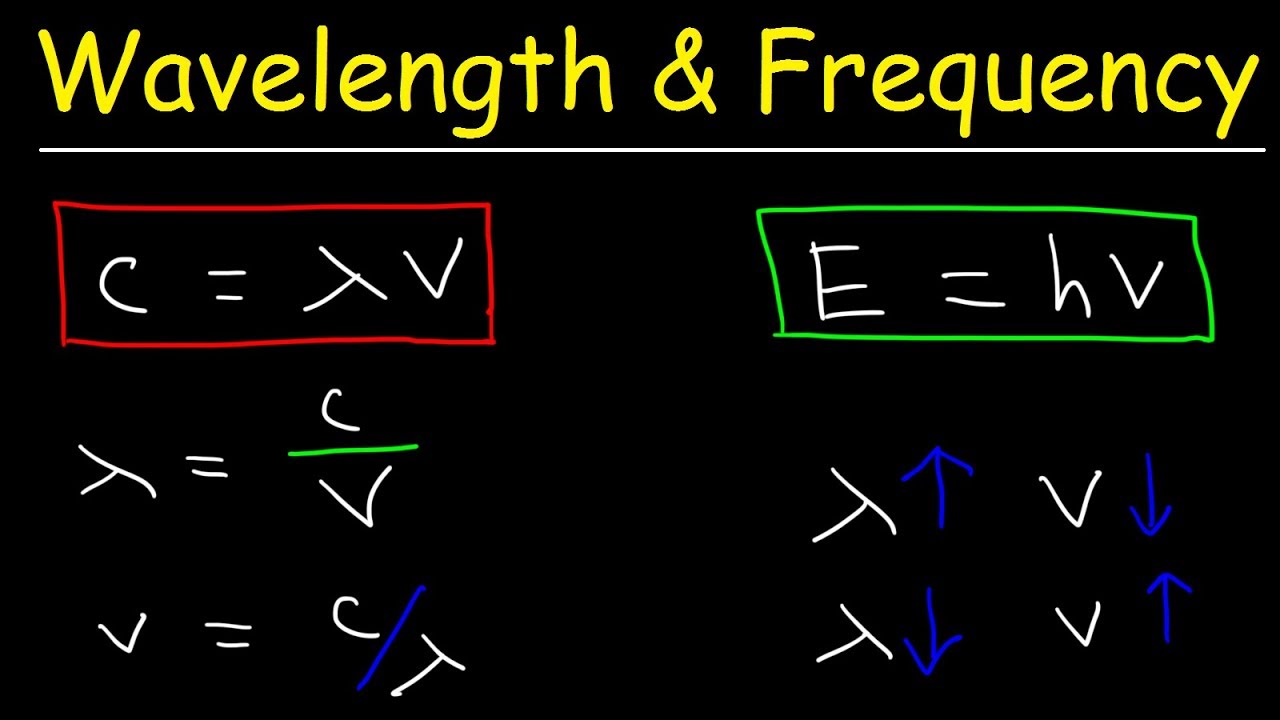
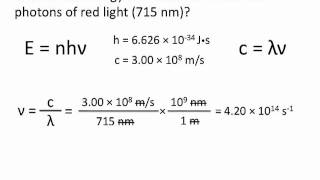
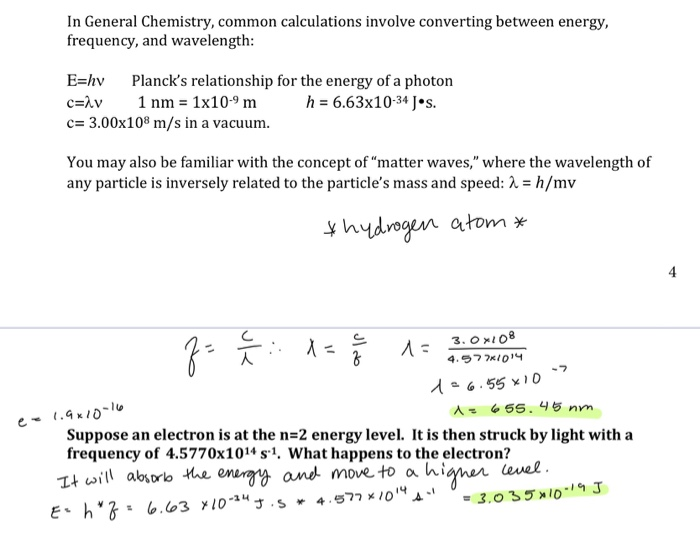
E hv chemistry. This formula is from chemistry. E is the energy of electromagnetic radiation h is the planck constant and v is the frequency of electromagnetic radiation. Where e energy in j or calories h planks constant 6 022 x 10 34. Energy is equal to planck s constant times nu the greek letter.
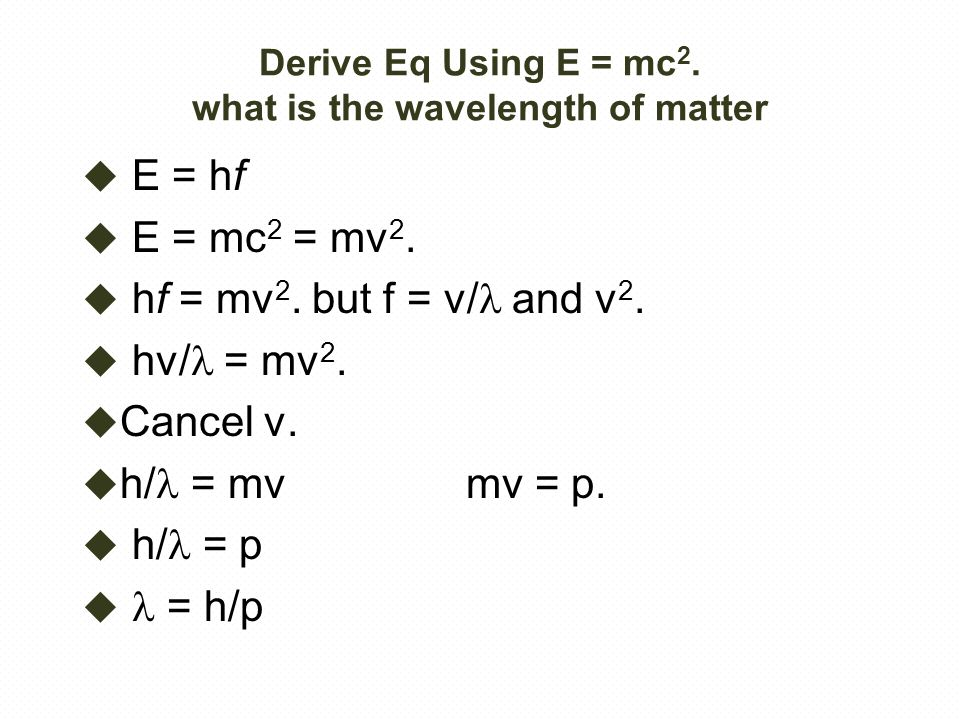
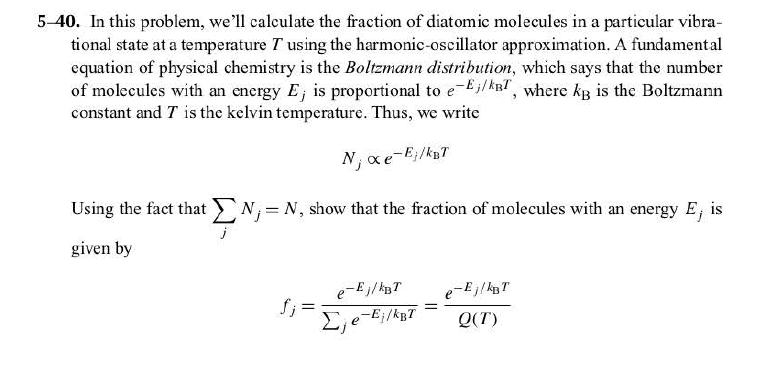
H is planck s constant and is. E energy of the photon. The equation above is a fundamental equation to spectroscopists. In 1900 planck introduced the hypothesis that energy is emitted in quanta instead of a continuous emission.
6 626 068 96 33 10 34 joules seconds.