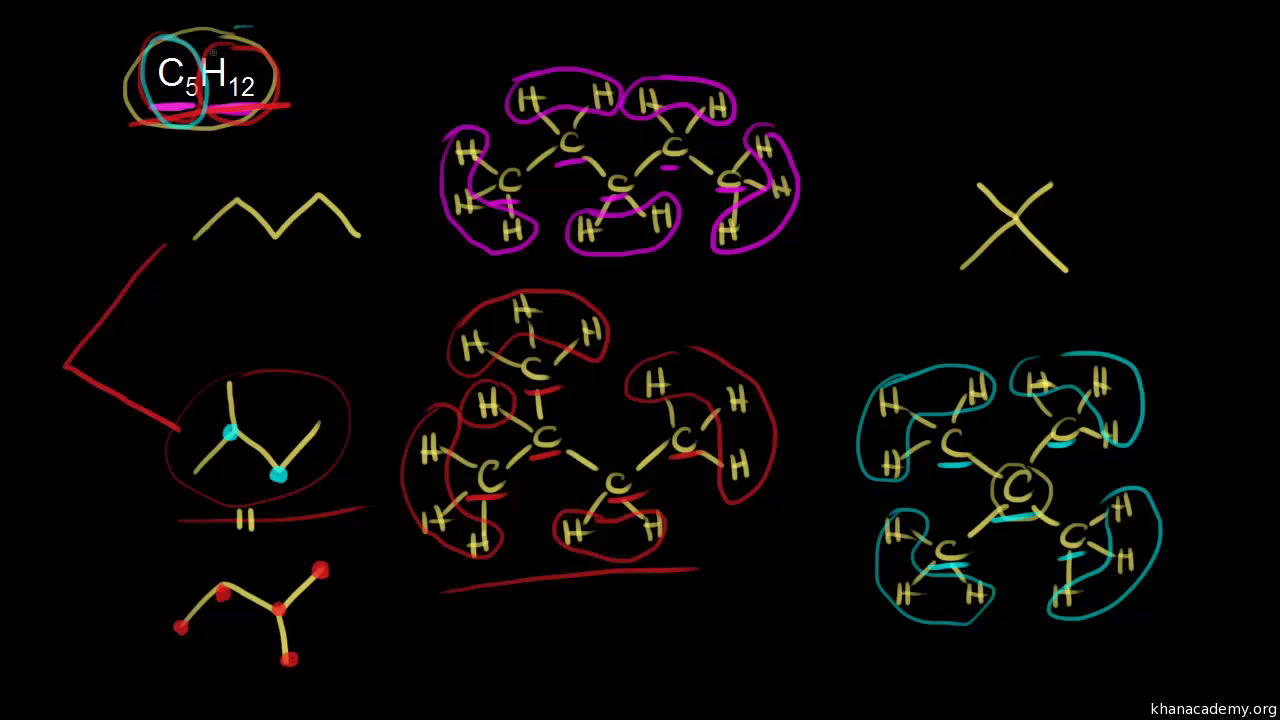
C3h8o Structural Isomers
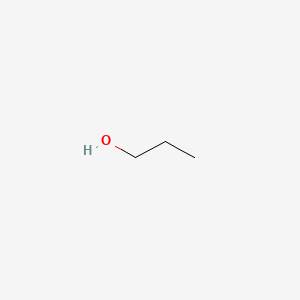
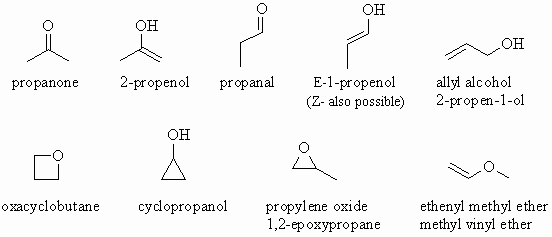
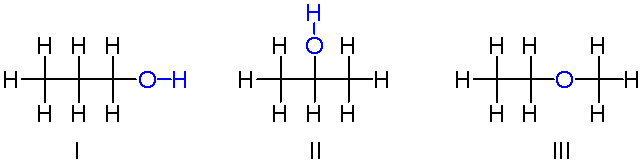
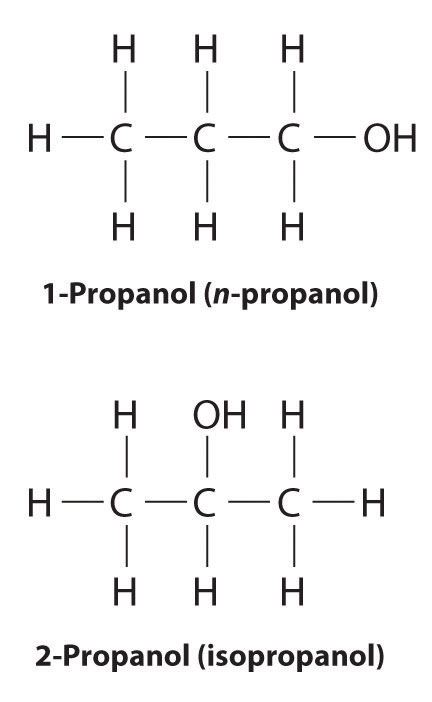
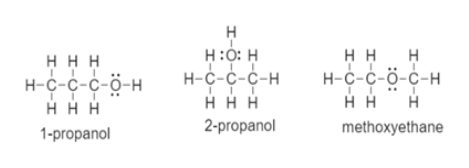
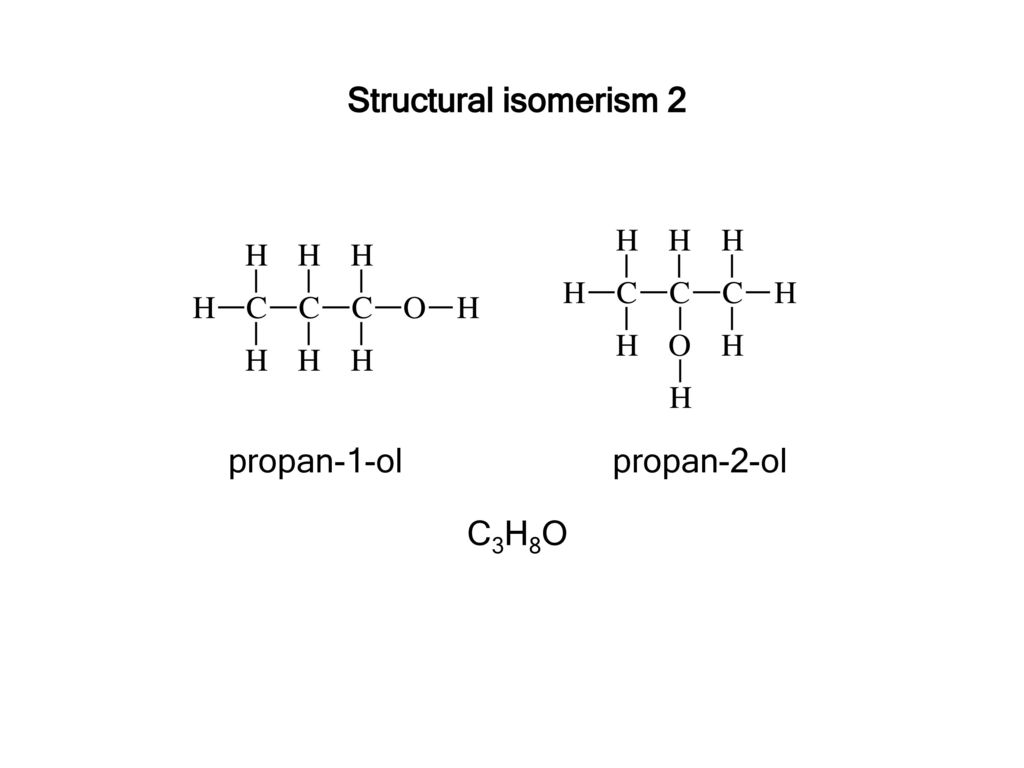
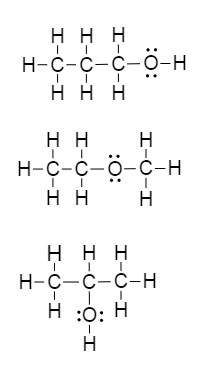
The isomers for the molecular formula c3h8o include methoxyethane propan 1 ol and propan 2 ol.
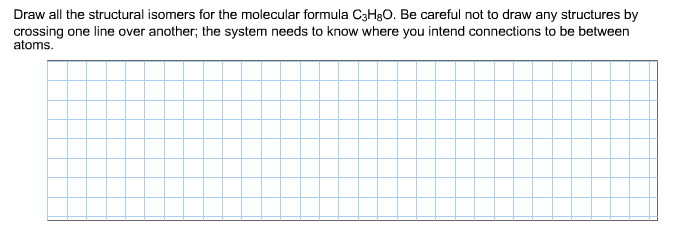
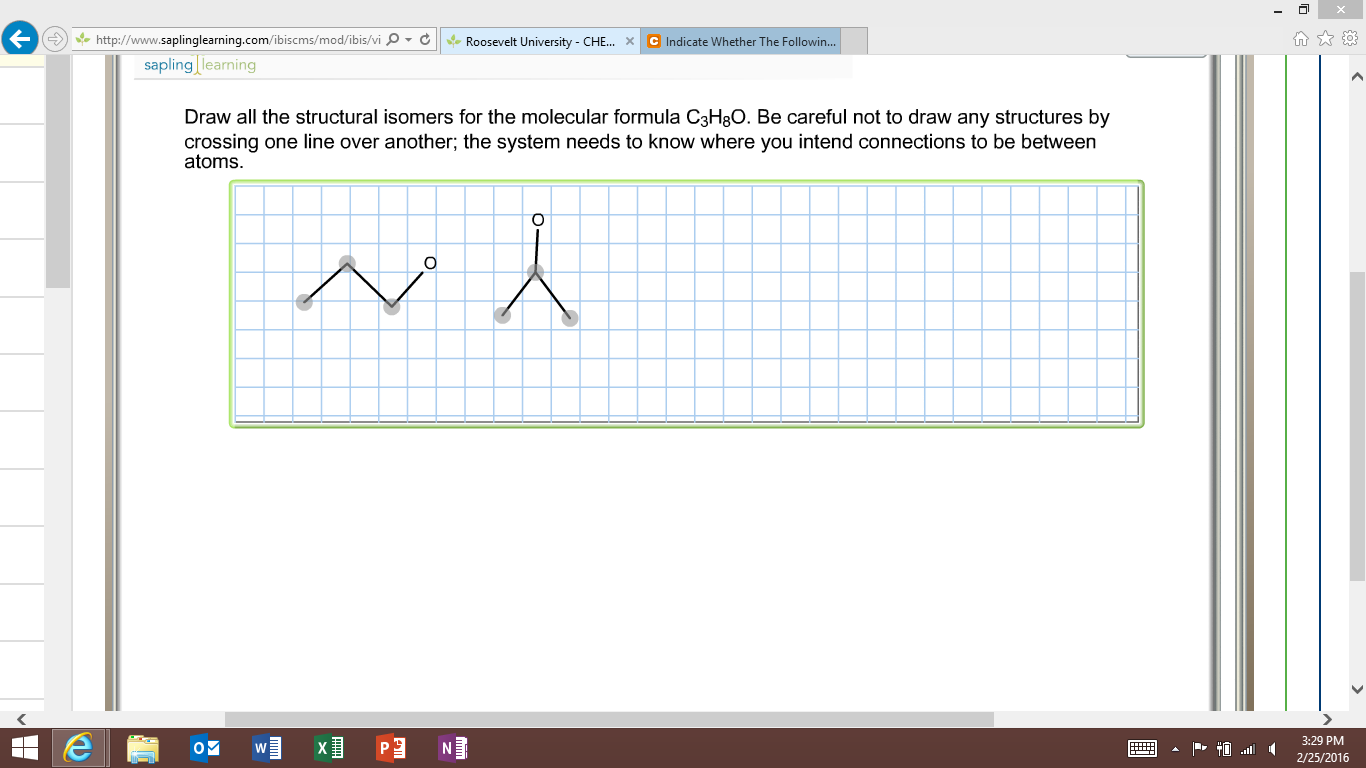
C3h8o structural isomers. Ch 3 ch oh ch 3. Ethyl methyl ether c3h8o cid 10903 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more. Be careful not to draw any structures by crossing one line over another. It has the same connections.
Rus 53 1 1998 43 49 in original 43 49 nist spectra nist ri. And as you go further in organic chemistry you ll learn that the first two isomers we talked about so this one and this one the ones that have an oh on it those are called alcohols. Draw all the structural isomers for the molecular formula c3h8o. And the last structural isomer is called an ether.
Get the latest public health information from cdc. C3h 8o has three constitutional isomers. The molecular formula c3h8o may refer to. The first isomer methoxyethane is an ether and the other two isomers are alcohols.
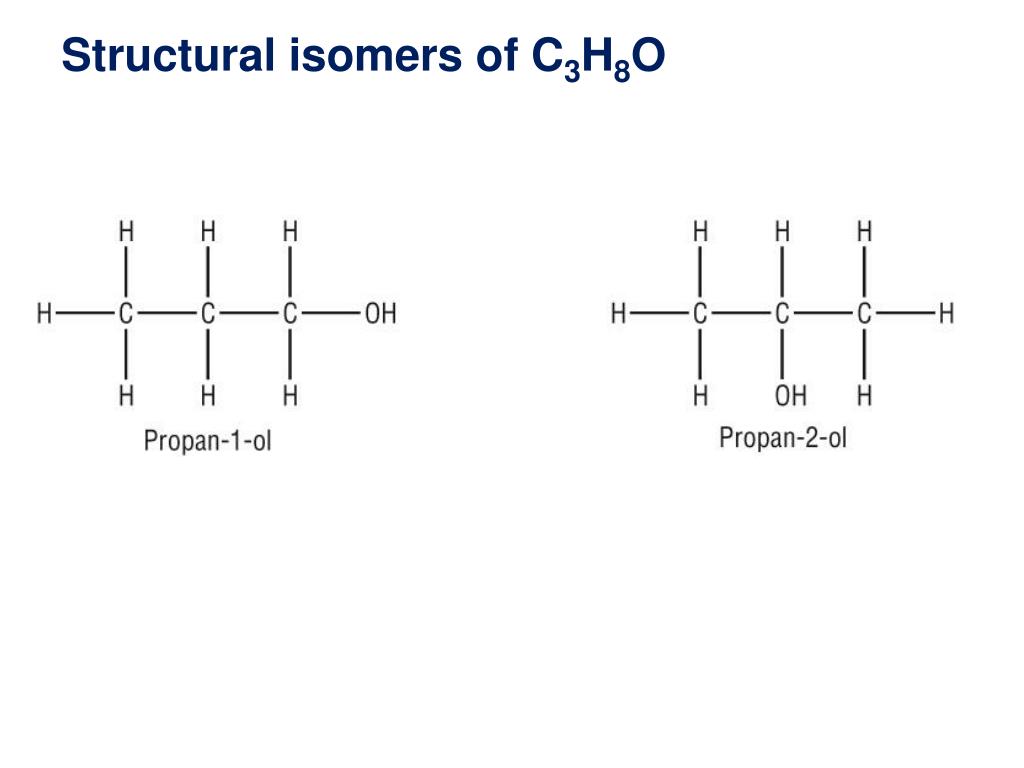
The first isomer methoxyethane is an ether and the other two isomers are alcohols. Isopropyl alcohol isopropanol 2 propanol ch 3 choh ch 3 cas number 67 63 0. Propanol c3h8o cid 1031 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more. Ch 3 ch 2 ch 2 oh.
The principle of structural analogy in the calculation of gas chromatographic retention indices using physico chemical constants of organic compounds zh. Covid 19 is an emerging rapidly evolving situation. Covid 19 is an emerging rapidly evolving situation. Get the latest public health information from cdc.
Methoxyethane is also called methyl ethyl ether and appears as a colourless liquid. Each isomer is obtained by arranging the atoms in the c 3 h 8 o molecular formula into a different structural formula. Jump to navigation jump to search. Ch 3 ch 2 o ch 3.
So we have a total of three structural isomers that have the molecular formula c3h8o. Each isomer is obtained by arranging the atoms in the c3h8o molecular formula into a different structural formula.