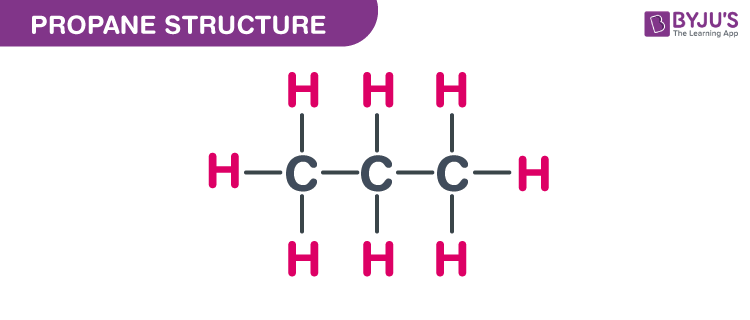
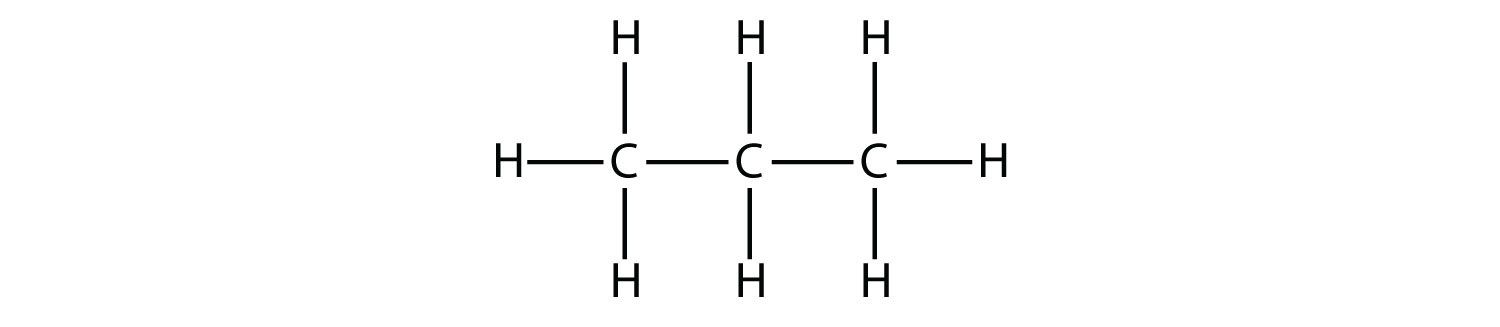
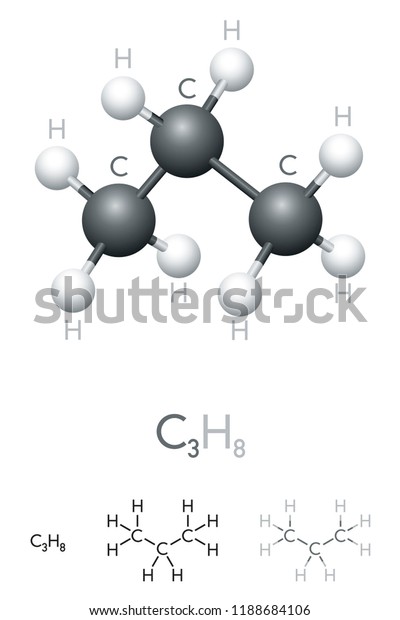
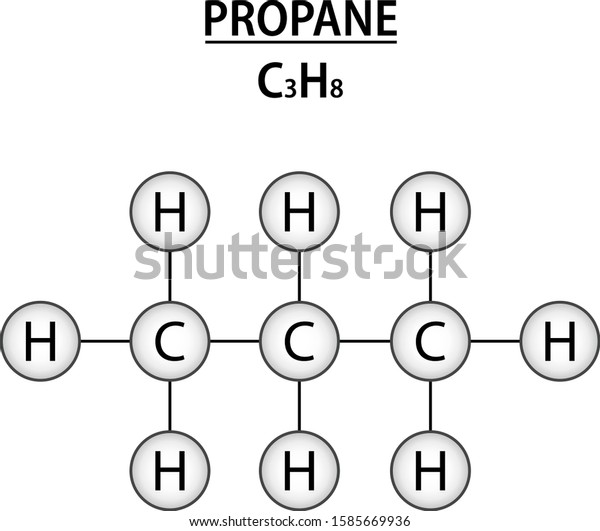
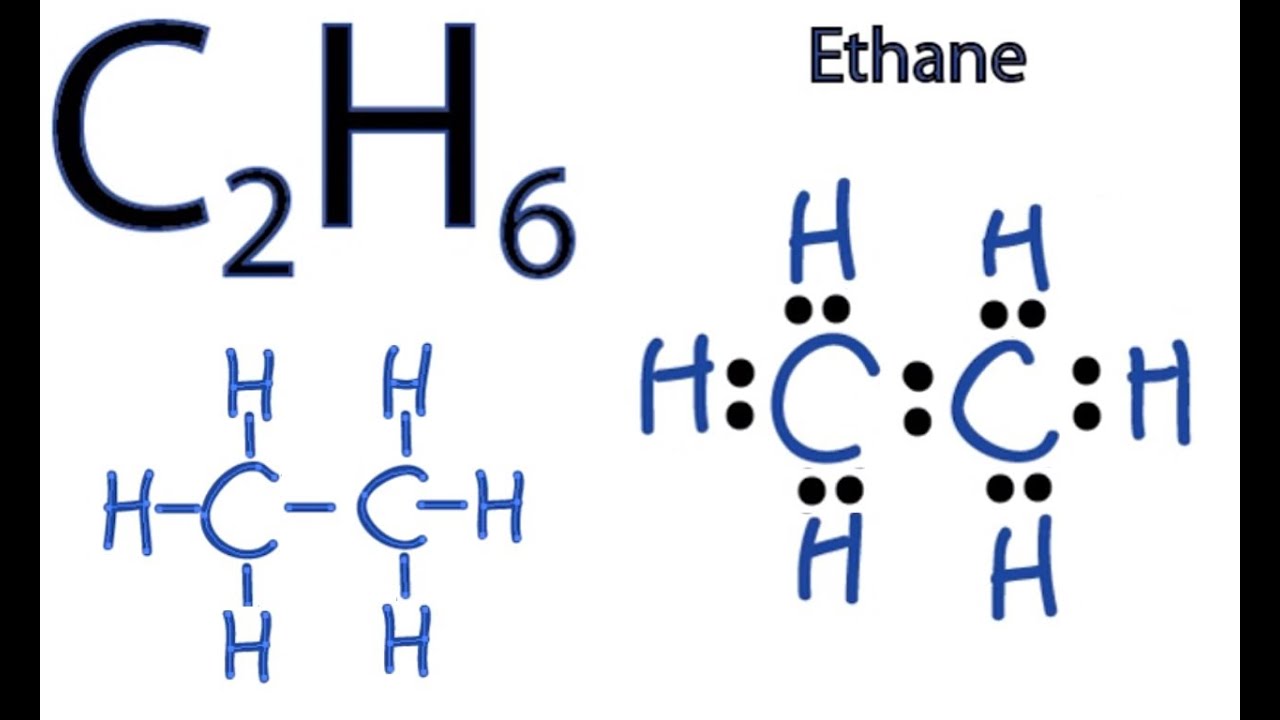
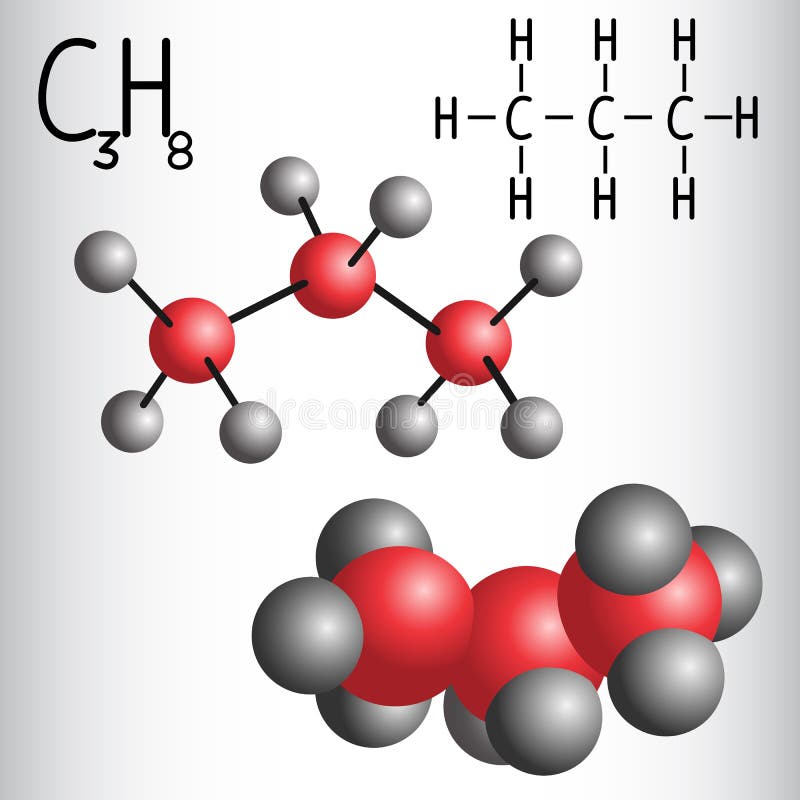
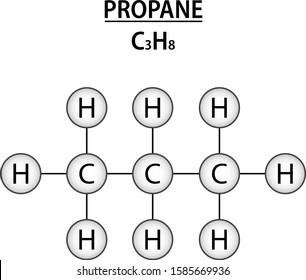
C3h8 Structure

The middle carbon has two hydrogens bonded to it while the two end carbons have three hydrogens bonded.
C3h8 structure. A step by step explanation of how to draw the c3h8 lewis structure propane. Its lewis structure is. Put hydrogen on carbons until octet is fulfilled. Alternatively a dot method can be used to draw the lewis structure.
Propane 13c3 c3h8 cid 12314537 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more. Get the latest public health information from cdc. So we ve satisfied the octets on each of the carbon atoms. C3h8 contains three carbon atoms and eight hydrogen atoms.
Each hydrogen has a single bond that s two valence electrons so its outer shell is full. Put cabons side by side as shoun below. The 2d chemical structure image of propane is also called skeletal formula which is the standard notation for organic molecules. So we ve used all 20 valence electrons for the c3h8 lewis structure.
Propane 74 98 6 107 00 6 627 21 4 1002 28 4 7651 40 3 90693 76 8 95722 07 9 361387 58 8. A by product of natural gas processing and petroleum refining it is commonly used as a fuel. Structure properties spectra suppliers and links for. Calculate the total valence electrons in the molecule.
The carbon atoms in the chemical structure of propane are implied to be located at the corner s and hydrogen atoms attached to carbon atoms are not indicated each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom. Lewis dot structure of c 3 h 8. It is a gas at standard temperature and pressure but compressible to a transportable liquid. And for the carbons each carbon has four single bonds so that s 8 valence electrons and that s an octet for the carbon.
Propane ˈproʊpeɪn is a three carbon alkane with the molecular formula c 3 h 8. Covid 19 is an emerging rapidly evolving situation.