C3h6 Lewis Structure Isomers

Distinction between c3h 6 isomers.
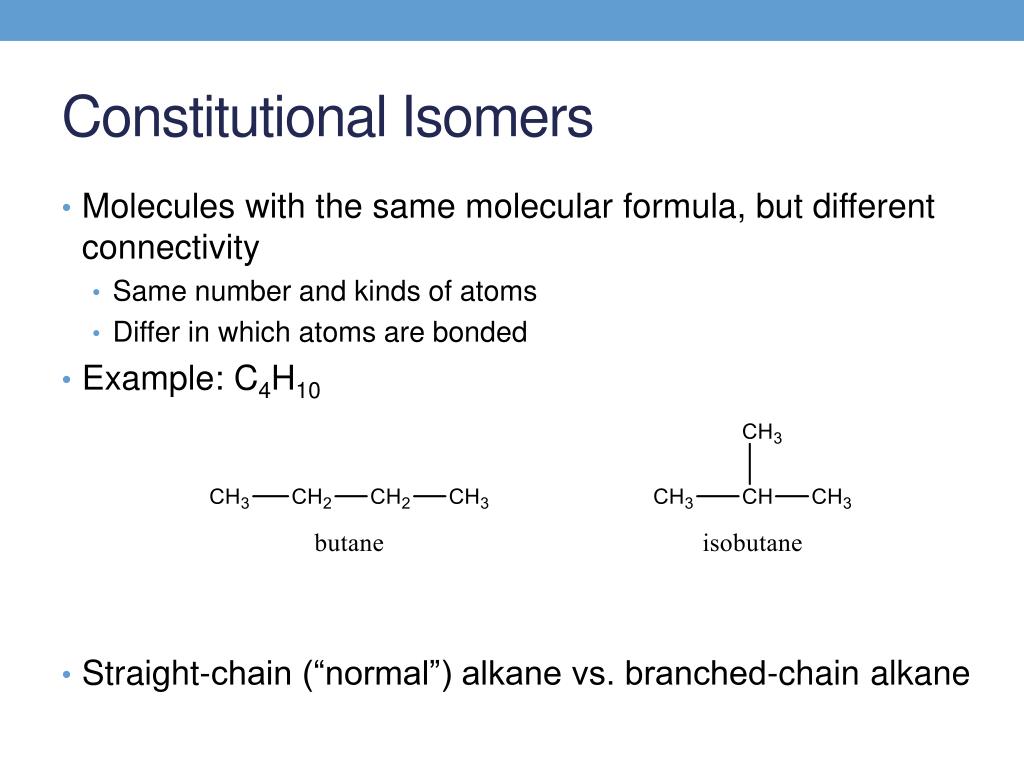
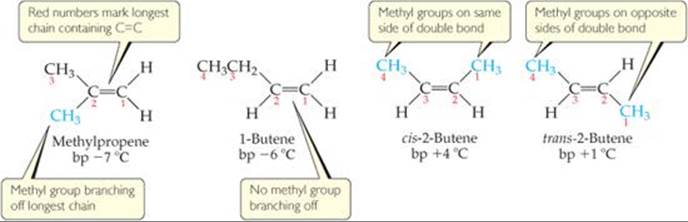
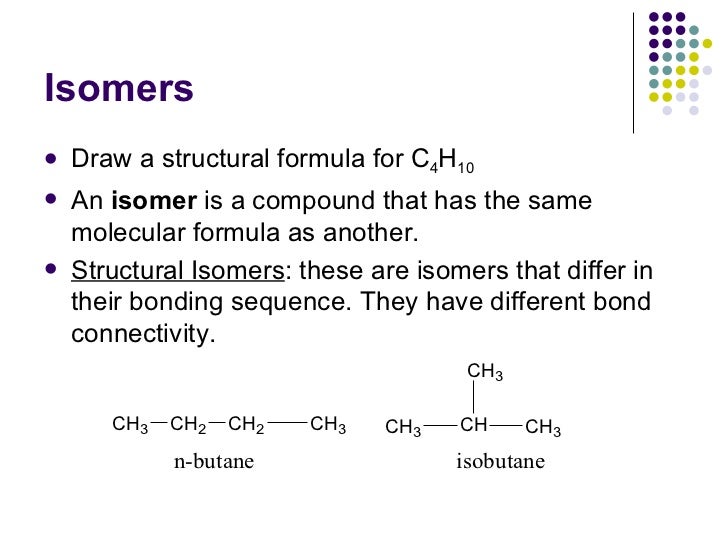
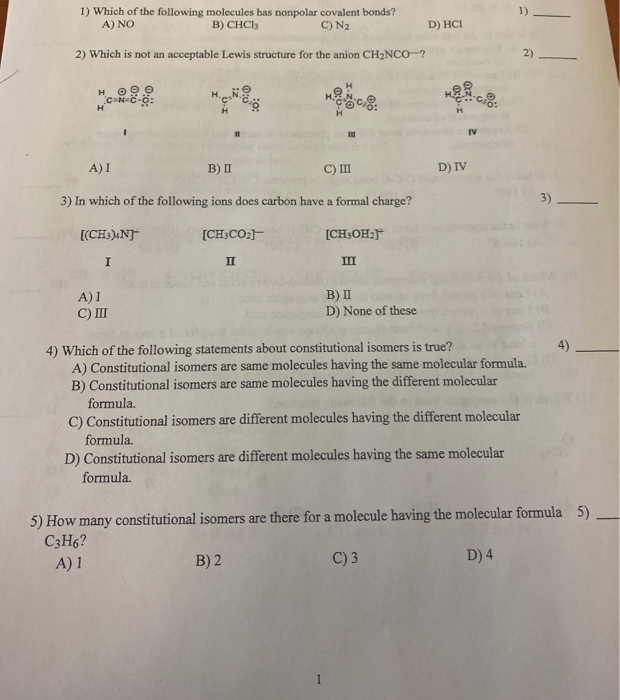
C3h6 lewis structure isomers. C3h6 isomers c3h6 isomers. Draw lewis structures for the two isomers with molecular formula c3h6. Structure and reactivity of organic radical cations. There are two constitutional isomers for the molecular formula ce c4h10.
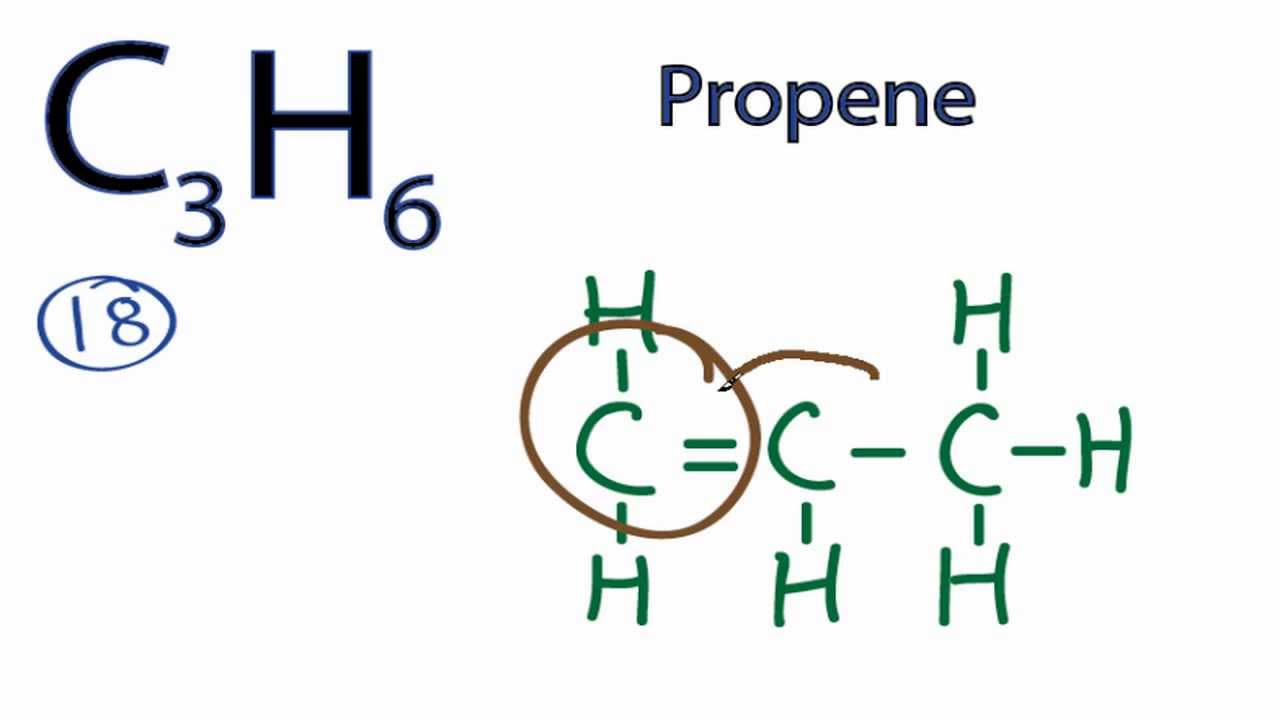
C2h4cl2 two isomers b. The thing about c3h6 is there s more than one way to draw it based on the chemical formula that we re given here. For c3h6 we have a total of 18 valence electrons. Solution for draw lewis structures for each molecular formula.
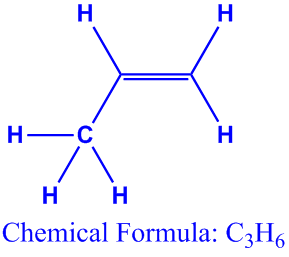
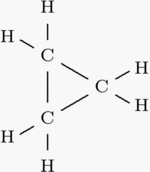
So let s look at the two ways you can draw the c3h6 lewis structure. Clicking on the figure will load an animation involving drawing lewis electron dot structures. The first structure is called cyclopropane. For this chemical formula there are two different ways to draw the c3h6 lewis structure.
Figure 4 demonstrates drawing lewis electron dot structures for these compounds. C3h8o three isomers c c3h6 two isomers. A step by step explanation of how to draw the c3h6 lewis structure. Constitutional isomers of ce c4h10.
1e 2e 3e 4e 5e 6e 7e 8e 9e 10e 11e 12e 13e 14e 15e 16e 17e 18e 19e 20e 21e 22e 23e 24e 25e 26e 27e 28e 29e 30e 31e 32e 33e 34e 35e 36e 37e 38e 39e. I think there are two isomers of c3h6. Ch1 ch2 ch3 ch4 ch5 problem. Reactions of cyclopropane and propene radical cations with water and methanol at low pressure.
Draw the lewis structures of two isomers of c 3 h 6. International journal of mass spectrometry and ion processes 1992 113 2 105 115. Describe a weakness of using lewis structures to model covalent bonds. One is propene ch2 chch3 and the other is cyclopropane looks like a triangle.