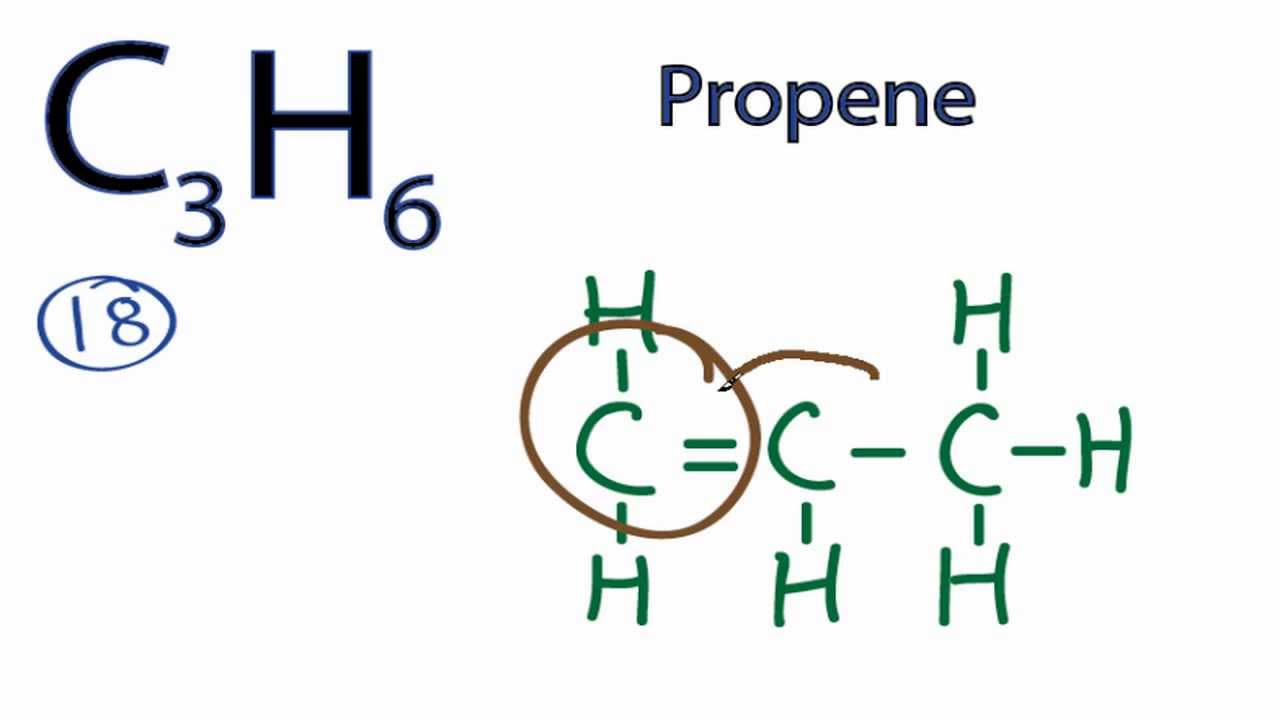
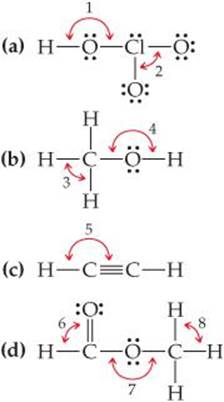
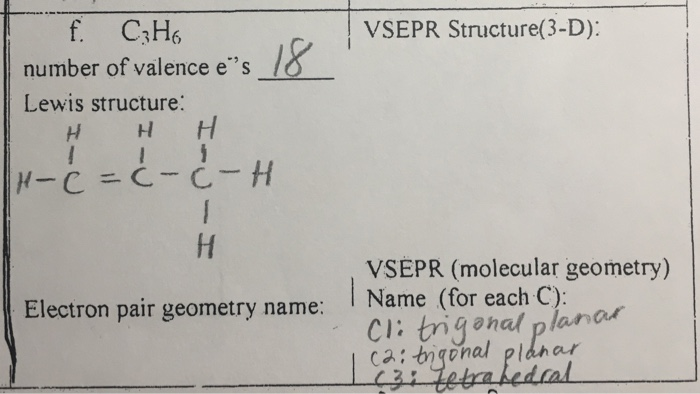
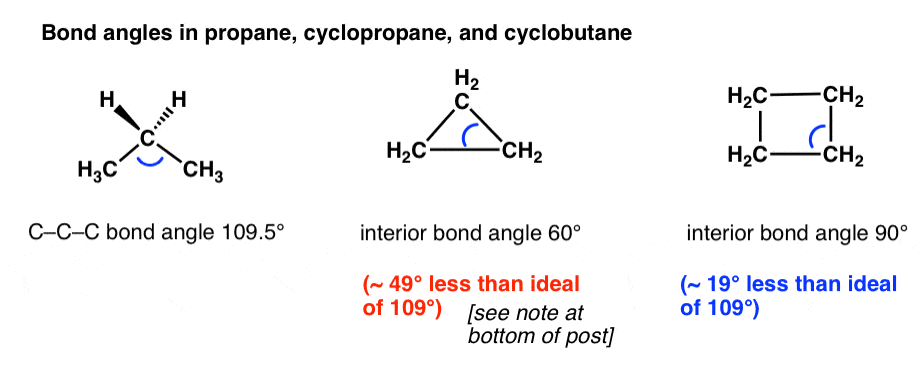
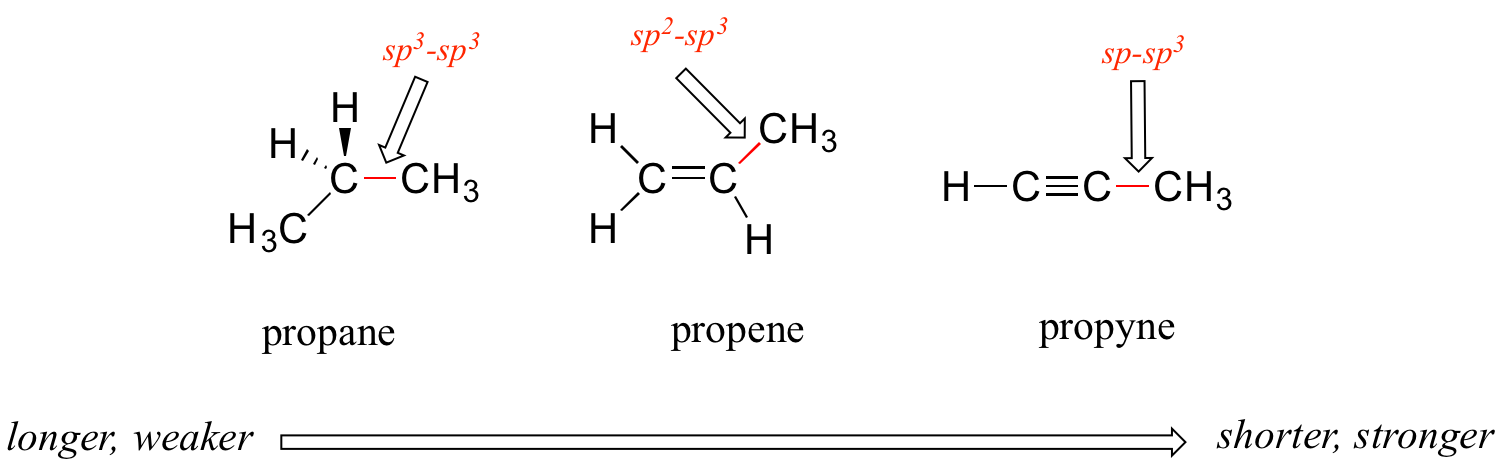
C3h6 Lewis Structure Bond Angles

A quick explanation of the molecular geometry of ch3oh methanol including a description of the ch3oh bond angles.
C3h6 lewis structure bond angles. Computed by pubchem 2 1 pubchem release 2019 06 18. A bonding orbital for n1 c2 with 1 9943 electrons has 62 86 n 1 character in a. Et first all angles in carbon atoms are tetrahedral. Consider the species bf type ax.
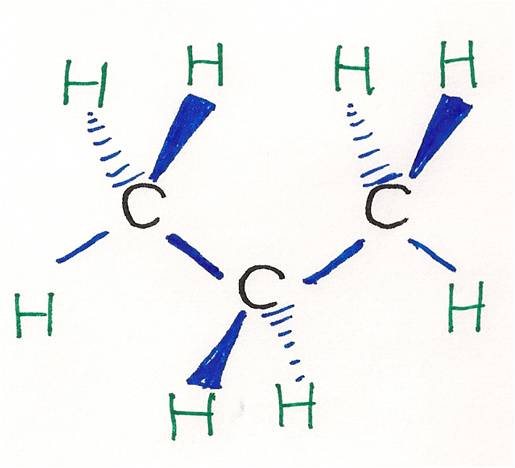
This is far less than the thermodynamically most stable angle of 109 5 for bonds between atoms with sp 3 hybridised orbitals and leads to significant ring strain the molecule also has torsional strain due to the eclipsed conformation of its hydrogen atoms. Hybridization in the best lewis structure. Hydrogen bond donor count. Since the lewis dot structure of this compound is a tetrahedral around the c atom that is and all four electron pairs are bonding pairs the ideal angles should be 109 5 degrees 0 0 1 0.
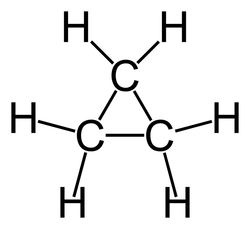
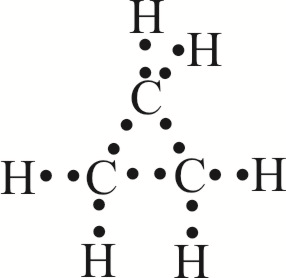
The bond angles between the carbons are 60 degrees while the bond angles between the hydrogen are 115 degrees. Looking at the ch3oh lewis structure we c. The picture to the right shows a lewis structure of cyclopropane. Computed by cactvs 3 4 6 11 pubchem release 2019 06 18 exact mass.
All of the b f bonds in bf3 since the f atoms are the same the molecule would would not be polar. The bond angles in this molecule aredegrees. The triangular structure of cyclopropane requires the bond angles between carbon carbon covalent bonds to be 60. This picture shows the individual atoms and their bonds where the picture below shows the bond angle measurements more clearly.
Please note that your structure can t be well described by a single lewis structure because of extensive delocalization. Angles between carbon atoms are 60 degrees two of electrons of each carbon atoms are bonds with two. The hybridization of the atoms in this idealized lewis structure is given in the table below. Computed by cactvs 3 4 6 11 pubchem release 2019 06 18 rotatable bond count.