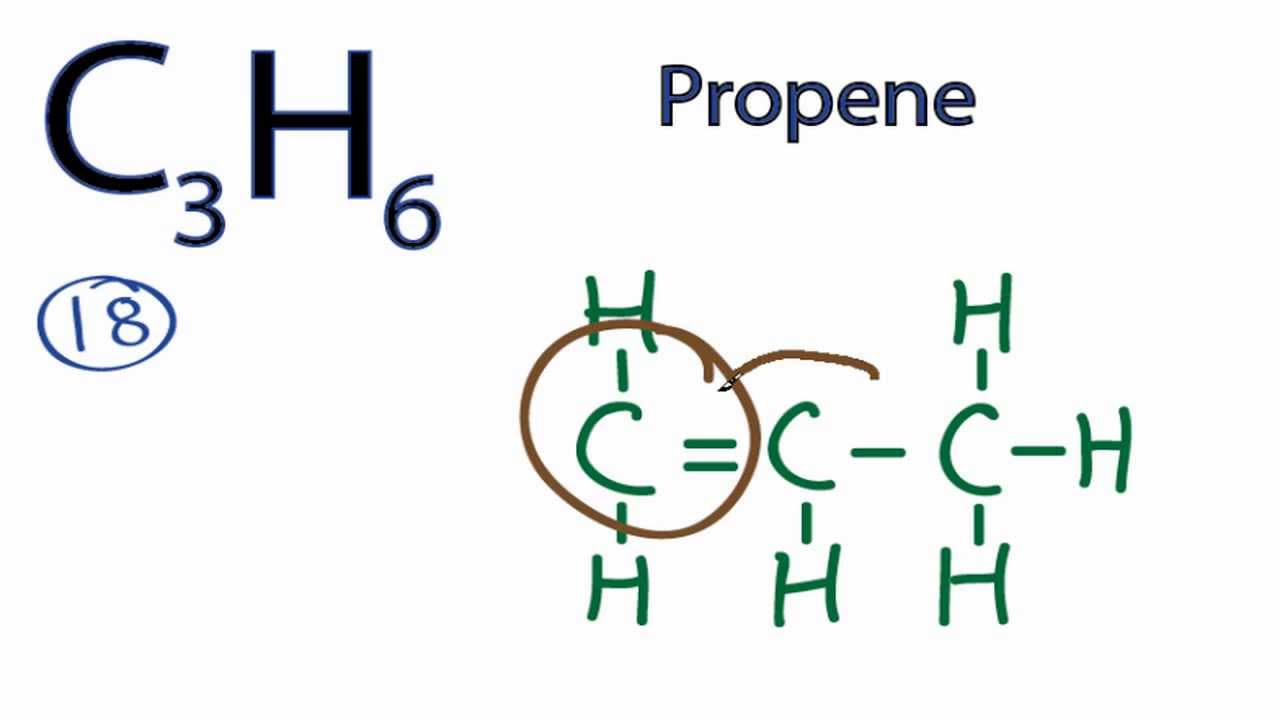
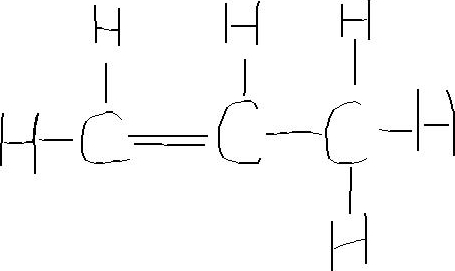
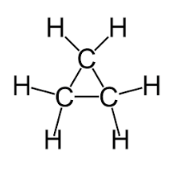
C3h6 Lewis Dot Structure
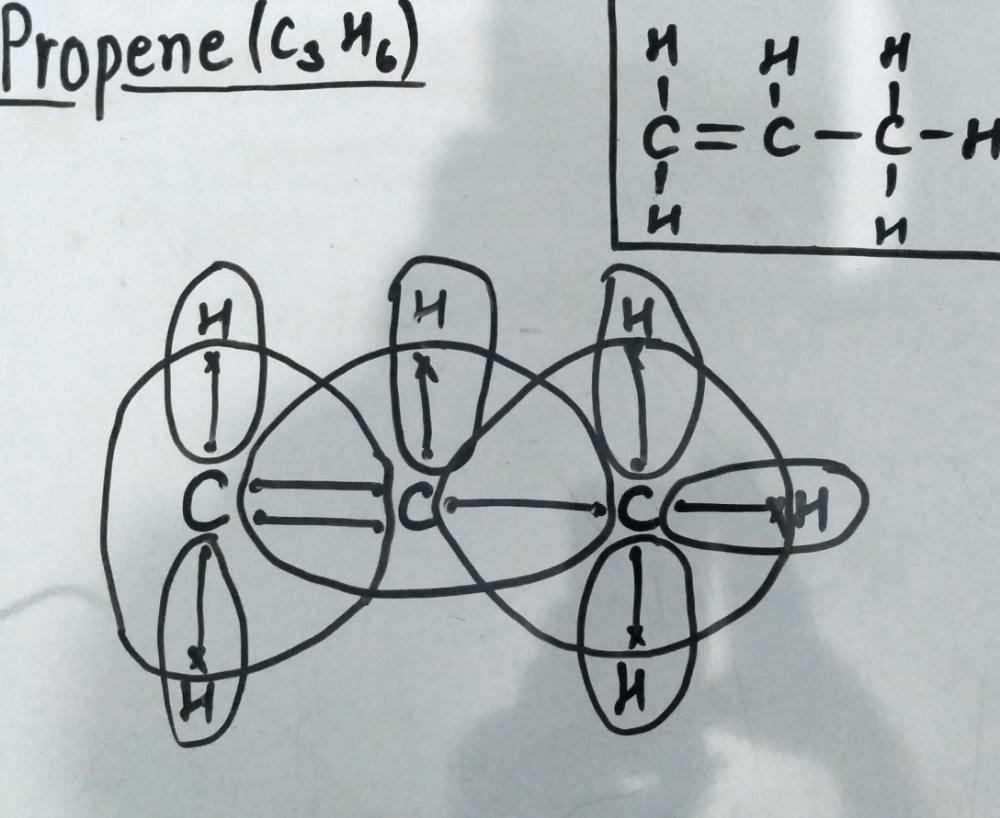
On the left c is a doubly bonded c atom with a pair of singly bonded h atoms.
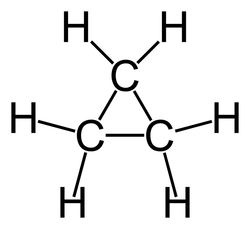
C3h6 lewis dot structure. There is no classic lewis dot diagram for c3h6. Second the molecule c3h6 is planar. Put cabons side by side as shoun below. This is the c3h6 lewis structure.
For c3h6 we have a total of 18 valence electrons. To save time chemists usually depict a bond as a line drawn from one atomic symbol to another. Et first all angles in carbon atoms are tetrahedral. Lewis publishers boca raton fl.
The first structure is called cyclopropane. The now center c has a singly bonded h. When we see cyclo we re thinking ring. Find more chemistry widgets in wolfram alpha.
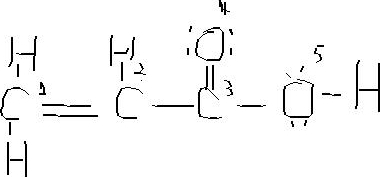
So let s look at the two ways you can draw the c3h6 lewis structure. The diagram is unusual. Calculate the total valence electrons in the molecule. Drawing each bond in a molecule as two dots gets old very fast.
Use information from step 4 and 5 to draw the lewis structure. Put hydrogen on carbons until octet is fulfilled. 137 hazardous substances data bank hsdb the pharmacokinetics of inhaled propylene have been investigated in male sprague dawley rats and cba mice in closed exposure chambers in which the atmospheric concentration time course was measured after injection of a single dose into the chamber atmosphere. The lewis dot structure for propene starts with two singly bonded c atoms.
Lewis dot structure of c3h6 1020821 ultra high vaccum experiments are routinely performed at a total pressure of 1x10 10 torr. The thing about c3h6 is there s more than one way to draw it based on the chemical formula that we re given here. Such representations are called lewis structures rather than lewis electron dot structures. Lewis dot structure of c 3 h 8.